Mechanism of Action Video, commonly known as MOA Video, visually explains how a drug or device acts to affect a physiological condition. It helps healthcare providers, doctors, and sometimes patients understand how a medication or treatment works.
Typically produced by healthcare marketing agencies on behalf of their clients, MOA videos are a standard part of any pharmaceutical company’s marketing toolkit. They’re regularly used in:
- Trade show booth displays
- Sales presentations by pharma reps
- Email marketing
- Online marketing
This guide includes everything producers or project and account managers need to know to produce MOA video. Plus, it covers tips to help you avoid mistakes that could have costly consequences.
Calculate Your Budget
Animation in general, and MOA videos in particular, can vary widely in price. The final cost depends on the studio you choose and the complexity of the revision process. However, you can calculate your production budget by considering a few common cost drivers.
4 Primary Cost Drivers Of Animated Moa Videos
- Style of Animation
- Length of Video
- Revisions
- Timeline
A good animation studio will explain the cost implications of decisions throughout the process. Here’s a closer look at how each of these cost drivers might impact your MOA video project.
1. Style of Animation
We discuss different styles of animation in more detail below. For now, it’s enough to know how they would rank in order from least to most costly:
- Whiteboard animation
- 2D animation
- 3D animation
Each style can be produced with varying levels of complexity, which can impact the overall cost. The more complicated the design, the higher your budget needs to be.
2. Length of Video
Longer videos require more hours of labor to animate, and higher investment. The shorter you can get your script, the more affordable your video will be to produce.
Aside from budget, there are other great reasons to try to keep your video short and focused. Consult with your team to figure out how much time the message will need. Remove anything that doesn’t directly support the main goal of your video.
3. Revisions and Approvals
MOA videos must go through a rigorous medical and legal approval process. Even in the best case scenario, you may need multiple rounds of revisions to get your video approved.
If you’re working with an outside studio, ask them how many revisions their quotes include. Depending on your (or your client’s) review process, you may need to budget more for reviews.
4. Timeline
If the project is given enough lead time, deadlines may not affect your budget. However, if a project starts late or has an aggressive timeline, costs may increase.
Communicate any important dates to your animation team. Let your animation team know as soon as possible if the schedule changes due to reviews or revisions
Choose an MOA Video Animation Style
An animated MOA video can be produced in many different styles, but 3D animation, whiteboard and 2D are the most popular.
3D Animation
3D animation is a very popular choice for an MOA video. Although it’s often the more expensive option, its ability to convey space and depth makes it popular for explaining scientific topics.
Curved shapes, such as molecules and cells, can look attractive in 3D. Additionally, the use of strong contrasting colors makes highly technical stories easier to follow.
Whiteboard Animation
Whiteboard animation is a popular style for MOA videos because of its clean and academic feeling, as well as being more affordable than many other styles. Additionally, making changes later on in production is easier to do in a whiteboard animation than more complex styles.
At IdeaRocket, we produce whiteboard animation digitally, which allows for not only for the effect of a hand drawing elements onto a whiteboard, but also for those elements to come to life and move around. The artwork is drawn by hand on a tablet so it can be easily integrated into a digital workflow.
Most of the industry uses the term “whiteboard animation” to describe this style, but sometimes it is also referred to as RSA video, RSA animation, quick-draw video, fast-sketch video, or fast draw video.
2D Animation
Less commonly used for MOA videos but still a viable option, is 2D animation. 2D animation techniques can be used to create fluid motion. If your video will include scenes with characters, which is rare for MOA but can be used to discuss patients and prescribing, 2D animation may be a good fit.
Additionally, 2D animation techniques can be used to enhance whiteboard animation as in the example below.
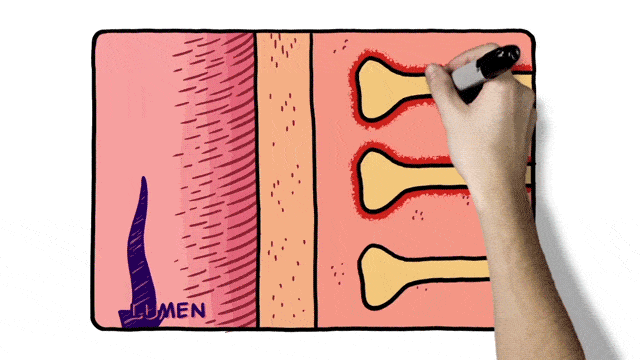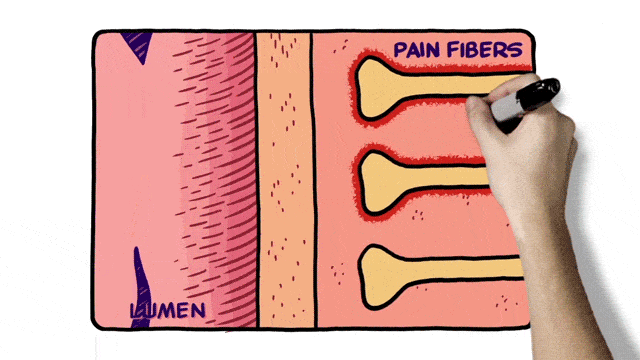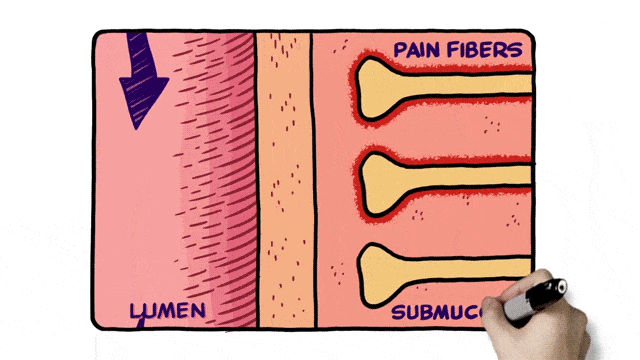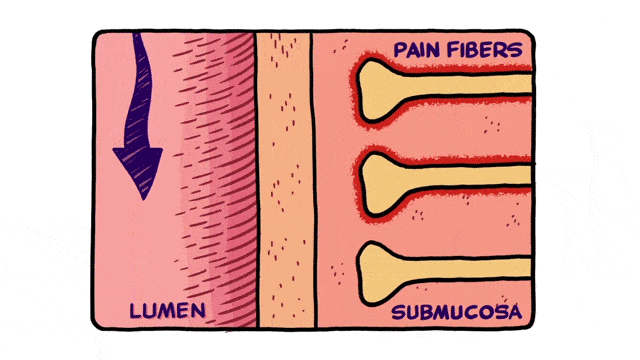
Select an Animation Company for MOA Video
Once you have a general idea of the cost factors and style you would like, you can start reaching out for quotes from animation providers.
Keep in mind that animation studios may not be familiar with the rigorous review process that an MOA video entails. Before choosing a studio to partner with ask:
- If they have worked with healthcare clients in the past
- Can they share examples of their previous MOA work
- What is their process for review and revision
- How would they handle scheduling complications resulting from legal and medical review
- Can you speak to some references
When shopping for animation providers, keep in mind that you often get what you pay for. Animation can be produced very cheaply — and very poorly — with modern technology, but the cost savings often aren’t worth it.
Aside from the reduced visual quality with a budget solution, many of these studios outsource the work overseas. As a result, they’re slow to respond to changes and you may find it difficult to even communicate what you need.
Producing an MOA video requires very clear and meticulous communication about medical details, phrasing, and terminology. Look for a studio with a proven track record and a strong portfolio.
Establish a Timeline
The amount of lead time needed for a project will depend on the studio you’re working with, the style of animation, and your legal review process. You’ll want to start pre-production (conceptual and scripting work) several months in advance of your final deadline.
Work directly with your studio when building a timeline for production. Find out how long they will need for the actual animation production, and then add in time for reviews.
If you can afford to, leave about six weeks between the wrap date of your production and the launch date of your video in case legal reviews require more iterations than you initially planned.
At minimum, you’ll want to make sure each of the following deliverables are cleared through legal:
- Script – should include all voice over and a description of the visuals, including any text that will appear on screen
- Storyboard – should include rough art
- Finished Animation – with voiceover and music
The medical/legal review process usually takes about 7 days. You may need to submit on a specific day of the week depending on the reviewer’s guidelines. Make sure to give your team enough time to review and request changes before submitting to legal.
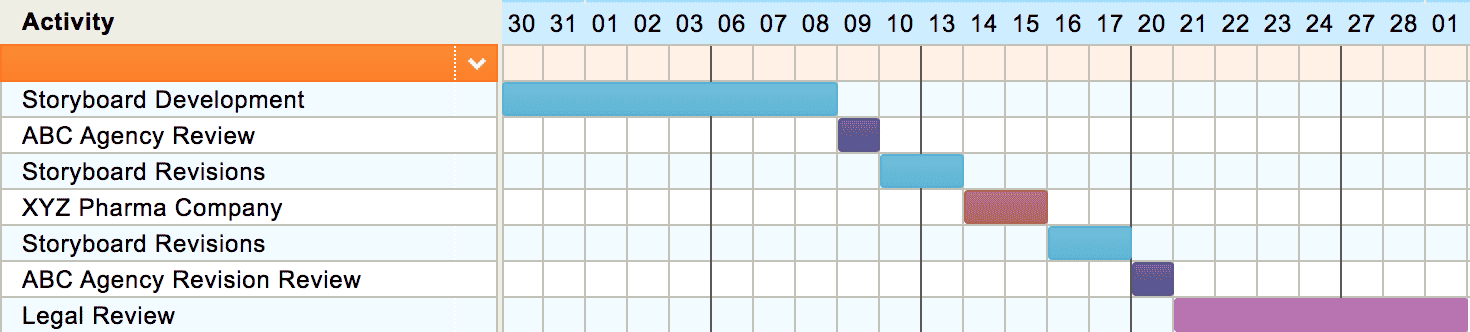
Below is a sample timeline for storyboard development, assuming that both an agency team (ABC Agency) and a client brand team (XYZ Pharma) will need to review the boards, and that items must be submitted for legal review by 10am on a Tuesday:
Create a Feedback Process
The easiest way to cut down on the number of iterations (and resources) a project goes through, is to have clear and consolidated communication with all of your stakeholders. One point person should be in charge of gathering feedback on each work-in-progress deliverable and delivering it to the video production team to implement.
Meetings are a good way to gather feedback and decisions from multiple individuals at once. If a synchronous session isn’t possible, give deadlines for feedback to keep the project on schedule.
Video review tools like Wipster and Frame.io can help you easily gather feedback from multiple teams. You can also invite stakeholders to use these tools so everything stays in one place.
When it comes to legal review, make sure to follow whatever process and format your company or client requires. You’ll likely need to move off the review tools at this stage.
To avoid overrunning deadlines, consolidate and address all marketing and brand team feedback before submitting assets for legal review. Even very small creative changes, such as altering phrasing in a voice over, can cause legal tangles.
Shepherd Your MOA Video Through Legal & Regulatory Review
The material below is intended for informational purposes only. It contains no legal advice or guidance, only tips for managing production alongside a team of legal professionals.
If you’ve produced an MOA video before, you’re likely familiar with the challenges in clearing the video through the legal review process. If you’re preparing to produce your first MOA video, trust us when we say that the process can take a lot longer than you think.
A seemingly endless amount of revisions from legal can drain your team and draw out production timelines, but there are a few things that producers and account managers can do to avoid this situation.
5 Ways to Avoid Setbacks During The Legal Review Process
- Know your submission guidelines and communicate them to your production team
- Avoid late-stage creative changes
- Understand which items most commonly get flagged for revision
- Get the Important Safety Information (ISI) approved separately
- Beware of copy/paste errors
Know Your Legal Review Submission Guidelines
Every company’s review process will be a little bit different. Frequently a legal team will require storyboards and other assets to be submitted in a specific format ( i.e. a certain number of panels per page, portrait vs landscape page layout, specific font point size, etc.).
Although boards can always be reformatted, doing so creates unnecessary work and increases back and forth between marketing and production teams. Every change introduces a new risk of human error. It’s best to find out exactly what the format is and share an example with your team at project kickoff.
Likewise, there may be requirements for how the completed video must be submitted. Some legal teams are okay with reviewing videos hosted on sites like Vimeo, others require a video file. Make sure your team is prepared to deliver the requested format.
This is particularly important to let your team know if assets need to be submitted for review at a particular time. If the team finds out about requirements late, they might not be able to meet the deadline and you’ll have to wait for the next review window.
Avoid Late Creative Changes
We often say that animation production is much like building a house: you start with the foundations (script), build the walls (art), and then put up the roof (animation). You don’t want to be still tweaking the script while artwork is being produced, just like you wouldn’t want to be making alterations to the foundation while you’re putting up the walls.
In a project with strict legal reviews, make sure each production item is locked before moving forward. Every adjustment could trigger the need for a new legal review.
Some changes are less likely to cause issues than others. The color of a character’s shoes wouldn’t likely set off any legal/regulatory alarms (unless they start to look like a patented product, but that’s a whole other can of worms…). Changing how the risks of a drug are explained most definitely will. See the section below for some tips on recognizing items that are commonly flagged.
The best way to avoid late changes is to make sure that everyone that needs to weigh in — both agency creative and client brand teams — has ample time to review an asset before it’s sent to legal.
Understand Common Items That Get Flagged For Revision
If you understand what aspects of a video are most sensitive, you can guide your team to avoid making late changes on those components.
The FDA has released a short video to help advertisers understand the most common pitfalls:
Beyond the videos, helpful top-level information can be found on the OPDP section of the FDA website, or in their Bad Ad Program page.
You can contact the FDA directly to ask questions. We did it for the purposes of writing this guide and are happy to say that the representatives we spoke to were helpful and friendly.
Although much of the information in these links is related to consumer-facing promotional materials, many of the same regulations apply to materials catering to a professional healthcare audience as well.
Here are a few common violations the FDA lists in the above linked video:
- Overstating a drug’s efficacy
- Omitting or minimizing information about risks
- Unsubstantiated superiority claims
- Unsubstantiated claims of efficacy or safety
Another item of importance is the “Fair Balance Requirement” a definition of which can be found on the FDA’s Drug Advertising Glossary of Terms:
“The law requires that product claim ads give a “fair balance” of information about drug risks as compared with information about drug benefits. This means that the content and presentation of a drug’s most important risks must be reasonably similar to the content and presentation of its benefits.
This does not mean that equal space must be given to risks and benefits in print ads, or equal time to risks and benefits in broadcast ads. The amount of time or space needed to present risk information will depend on the drug’s risks and the way that both the benefits and risks are presented.”
As a producer or account manager, understanding how to meet this requirement is likely to be beyond the scope of your responsibilities. That’s one of the reasons a legal team needs to review promotional materials before they go into use.
What you should know is that any time a creative team alters the way a drug’s efficacy or risks are presented in the MOA video, that video will need to go back in for legal review. Whenever possible, try to steer the team away from making changes to these aspects of a video after they have already been approved.
Get the ISI Approved Separately
The Important Safety Information (ISI) for an MOA video is a collection of information about the most important risks of a drug. It’s required by law to accompany the MOA video, and will be under heavy scrutiny in the legal review process. It usually appears as scrolling or sliding text at the end of the video and has very little (or nothing) to do with creative presentation or animation aside from possibly font choice or decorative lettering in the titles.
If possible, find out if the legal team can review and revise the ISI separate from the animated video. This would allow the reviews and revisions to begin almost immediately after script approval. Then the ISI can simply be attached at the end for final review.
Look Out for Copy/Paste Errors in Text
Notes and feedback for MOA videos often comes from multiple teams and individuals. Every company and agency has a different method for communicating internally.
Large chunks of technical information are often copied and pasted from one document to another as it changes hands. For the sake of accuracy and to eliminate human error, animators will copy this text directly into the programs they animate with.
It’s a good idea to keep an eye on special characters, footnotes, and subscripts as you pass information between teams. These are especially prone to errors when copying from one program to another.
Make an MOA Video the Easy Way
Even though making an MOA video sounds like an onerous process, it can also be highly rewarding. Careful planning and management can make production run more smoothly for everyone involved.
At IdeaRocket, we have ample experience producing videos for healthcare. We’ve made videos in techniques such as 2d and 3d animation, mixed media, whiteboard, motion graphics and live action. Let us know if we can help!